
Kavisar International Pharma
Merchant Exporter & Regulatory Consultancy
Empowering Healthcare Worldwide through Quality Manufacturing and Regulatory Excellence.
Kavisar International Pharma Pvt Ltd
Welcome to KaviSar International Pharma
Your Trusted Partner in Global Healthcare Solutions
At KaviSar International Pharma Pvt. Ltd., we are committed to improving lives by delivering high-quality, affordable, and innovative pharmaceutical products across the globe.
With a passion for excellence and a dedication to health, KaviSar has established itself as a trusted name in manufacturing, exporting, and supplying a wide range of human and veterinary formulations.


Who We are?
KaviSar International Pharma is a trusted name in the pharmaceutical industry, delivering WHO–GMP certified formulations that meet the highest international standards of quality, safety, and efficacy. We operate as a manufacturer, merchant exporter, wholesale distributor, and regulatory consultancy partner, providing a wide range of affordable, high-quality medicines along with end-to-end regulatory solutions to support global market access. Our mission is to empower partners with cost-effective pharmaceutical products and professional DRA (Drug Regulatory Affairs) services, helping them meet market demand and maximize growth.


Why Kavisar International Pharma?
1. Certified Quality : All products are manufactured in WHO–GMP certified facilities, ensuring compliance with global regulatory standards.
2. Extensive Product Portfolio : Wide range of formulations, including Tablets, Capsules, Syrups, Injections and more.
3. IHS Specifications & Regulatory Documentation : Formulations aligned with IHS specifications and supported with complete regulatory documentation for seamless international registration.
4. Competitive Pricing : Market-driven pricing ensures strong profit margins without compromising on quality.
5. Reliable Supply & Logistics : Fast and reliable delivery with consistent availability, global logistics support, and excellent after-sales service.


Our Vision :
To be recognized as a leading global pharmaceutical company known for quality, reliability, and ethical business practices, improving the quality of human life through trusted healthcare solutions.
Our Mission :
To manufacture and supply high-quality pharmaceutical formulations at competitive prices. To ensure global regulatory compliance and timely delivery for every market we serve. To promote innovation and continuous improvement in every stage of production. To build long-term partnerships with healthcare providers, distributors, and clients worldwide.


Our services
Kavisar International Pharma is your integrated partner for global pharmaceutical needs. We operate as a manufacturer, merchant exporter, and dedicated regulatory consultancy, providing a comprehensive range of affordable, high-quality medicines manufactured in WHO-GMP certified facilities. Our services are designed to maximize your market access and growth potential by ensuring product quality, regulatory compliance, competitive pricing, and reliable global supply.
Domestic
We ensure a steady and reliable supply of high-demand, quality pharmaceuticals across the Indian domestic market.


Global Export
We specialize in exporting a wide range of formulations to global markets (Africa, Asia, CIS, GCC).
Access end-to-end regulatory affairs services, including CTD/ACTD dossier compilation, DMF writing, and market-specific submissions.
Drug Regulatory Affairs






Govt. Tender
We are a trusted partner for public sector procurement, supplying essential medicines for Government Tenders and healthcare access projects.
Our Products Categories
We specialize in a wide array of therapeutic categories including: Antibiotics & Antibacterials, Analgesics & Antipyretics, Cardiovascular Drugs, Gastrointestinal Medicines, Antifungal & Antiviral Agents, Nutraceuticals & Dietary Supplements, Veterinary Formulations, Medical Devices & Topical Preparations, All our products are developed with precision, purity, and patient safety in mind.
Pharmaceuticals
As a leading pharmaceutical manufacturer and exporter, this category represents the cornerstone of Kavisar International Pharma.


Nutraceuticals
Kavisar International Pharma extends its expertise into the growing field of Nutraceuticals, offering a range of health-enhancing products.
Recognizing the critical role of animal health, Kavisar International Pharma offers a specialized range of high-quality Veterinary products.
Veterinary





Medical Devices
Kavisar International Pharma has diversified into the supply of essential Medical Devices and disposables vital for clinical and surgical settings.
Our Products Range
Kavisar International Pharma offers a comprehensive selection of pharmaceutical formulations designed to address a wide range of therapeutic needs. Our commitment to quality, efficacy, and safety is embedded in every product, from sterile injectables to specialized external applications. We ensure that our entire product range adheres to stringent international guidelines, making us a trusted partner for healthcare providers worldwide.
Dry Injectable
Our Dry Injectable range encompasses a critical segment of essential medicines, including antibiotics, antifungals, and other life-saving compounds.


Liquid Injectable
Kavisar International Pharma’s Liquid Injectable products provide immediate therapeutic effects, crucial for emergency and critical care scenarios.
Our range of Eye, Ear, and Nasal Drops are formulated with utmost care to provide targeted relief and treatment for delicate sensory organs.
Eye Ear & Nasal Drops


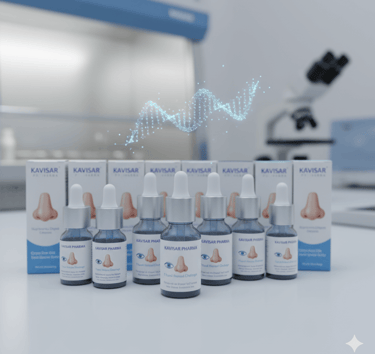


Eye Ointments
Our range of Ointment are formulated with utmost care to provide targeted relief and treatment for delicate sensory organs.
We Are Doing All These Countries
News & Udates
Get in touch


Our clients
Subscribe to our newsletter
Enjoy exclusive special deals available only to our subscribers.
Kavisar International Pharma Pvt. Ltd.
Trusted Pharmaceutical Merchant Exporter & Regulatory Consultancy
Address: 1, 17 The Central, New Survey Road, Survey Chowk, Dehradun – 248001, Uttarakhand, (India).
Email: info@kavisarinternationalpharma.com
Contact: +91 7017752681
Kavisar International Pharma - Merchant Exporter & Regulatory Consultancy | © 2025 All Rights Reserved


